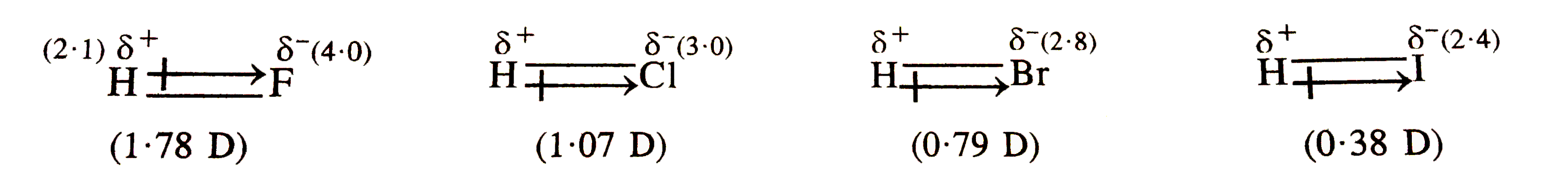Dipole Moments For Hi
March 24, 2025
Why does HCl have lower boiling point than HBr or HI, considering that HCl is much polar bond than HBr and HI? It would have dipole-dipole interaction, which is stronger than Van Between H2O and HF, which has more dipole moments, and why? - Quora molecule is said to be a “polar” - ppt download Dipole Moments For Hi