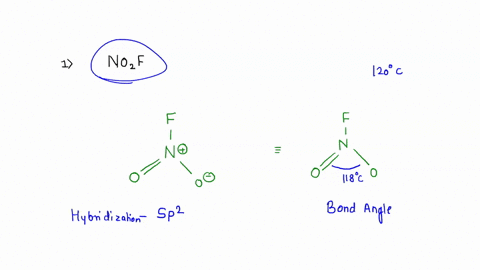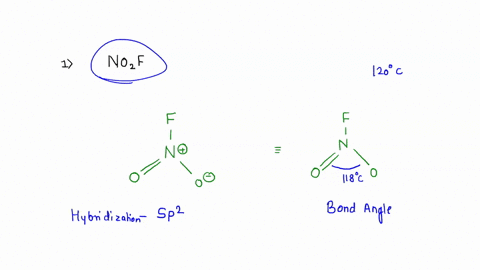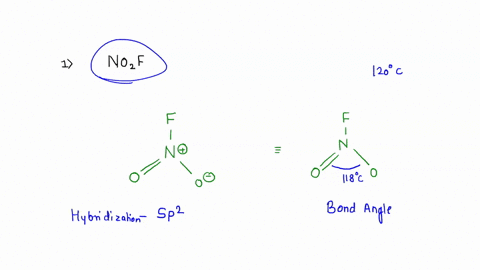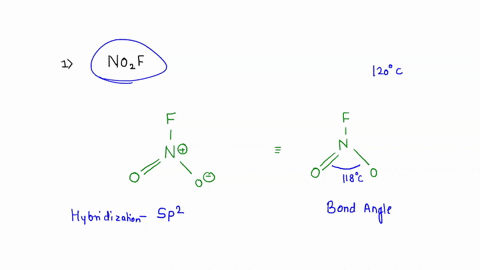
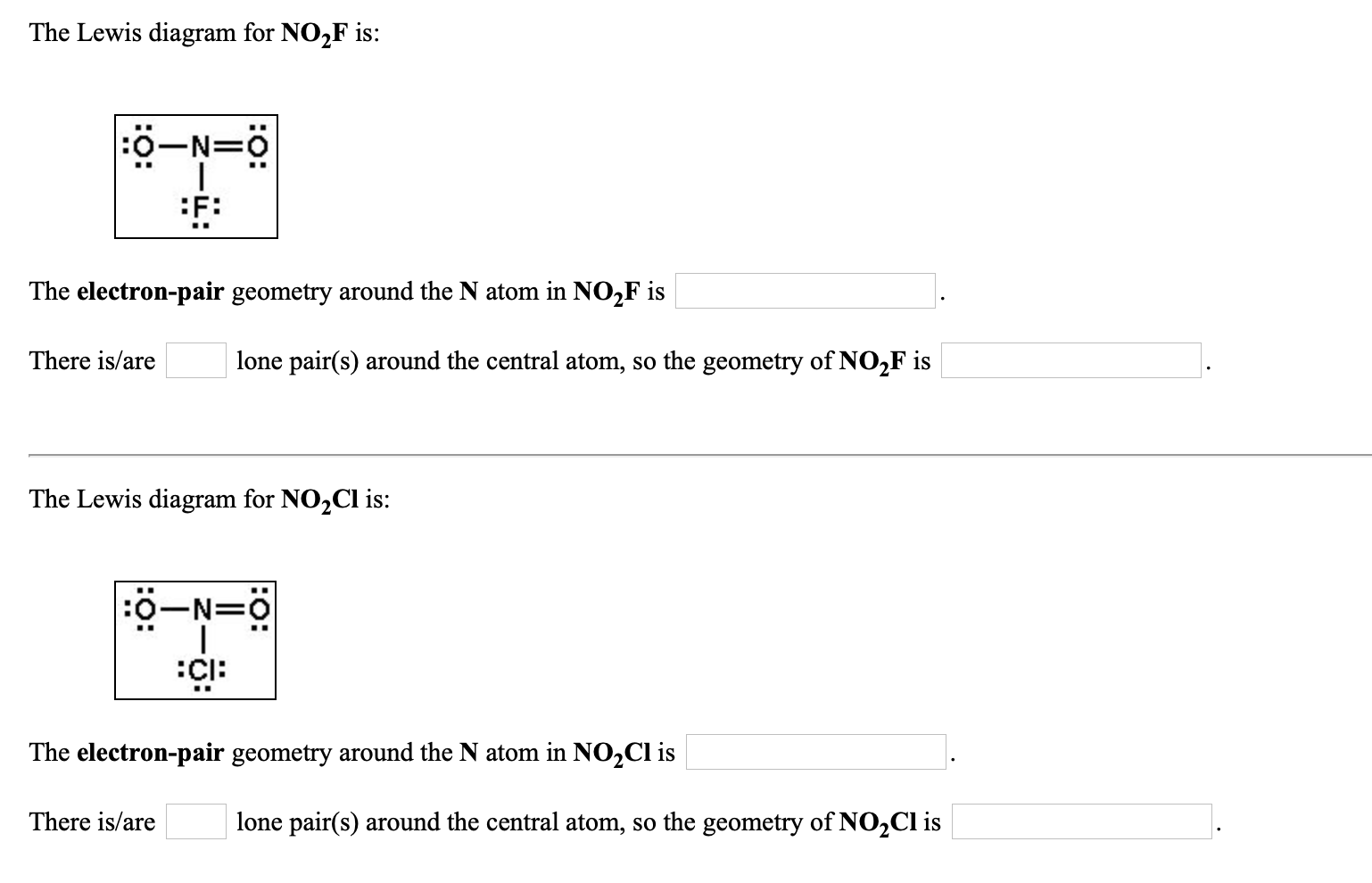
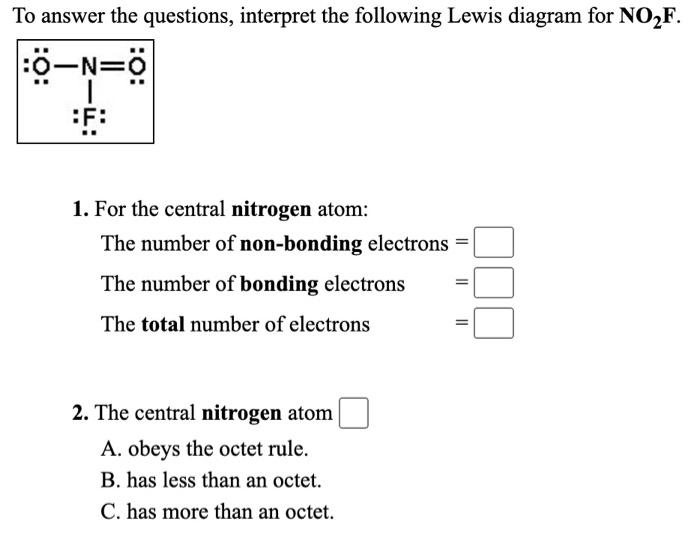
No2F
March 23, 2025
Draw all resonance structures for the nitryl fluoride molecule, NO2F. | Homework.Study.com Consider the following molecules or ions: BrOF3, ICl3, NO2F, and ClO2^- . Answer the following questions based on the Lewis structures and VSEPR theory prediction of their molecular shapes (a) Which one SOLVED: A. What is the hybridization of the central atom in NO2F? Hybridization: sp What are the approximate bond angles in this substance? Bond angles B. What is the hybridization of the No2F